Improved disease control
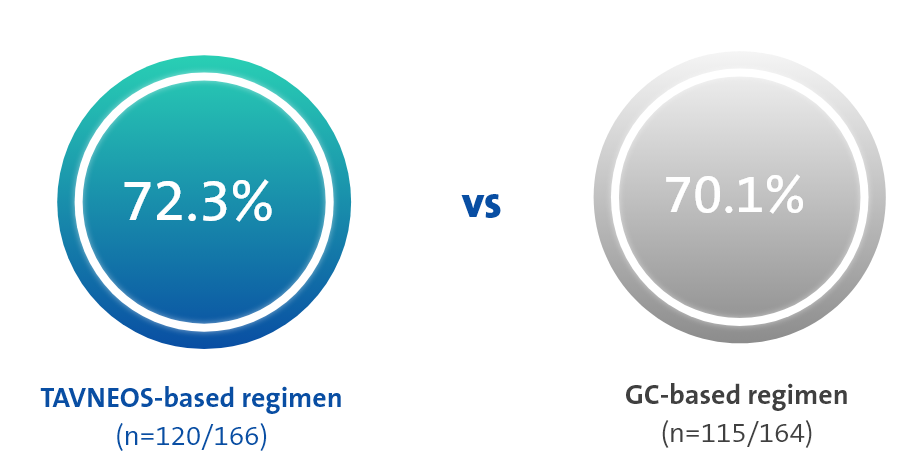
TAVNEOS-based regimen achieved non-inferior clinical remission at Week 26 and superior sustained clinical remission at Week 52 vs a GC-based regimen1*
TAVNEOS-based regimen achieved non-inferior clinical remission at Week 26 and superior sustained clinical remission at Week 52 vs a GC-based regimen1*
p<0.001 for non-inferiority
(Difference 3.4, 95% CI, -6.0 to 12.8)
p=0.007 for superiority
(Difference 12.5, 95% CI, 2.6 to 22.3)
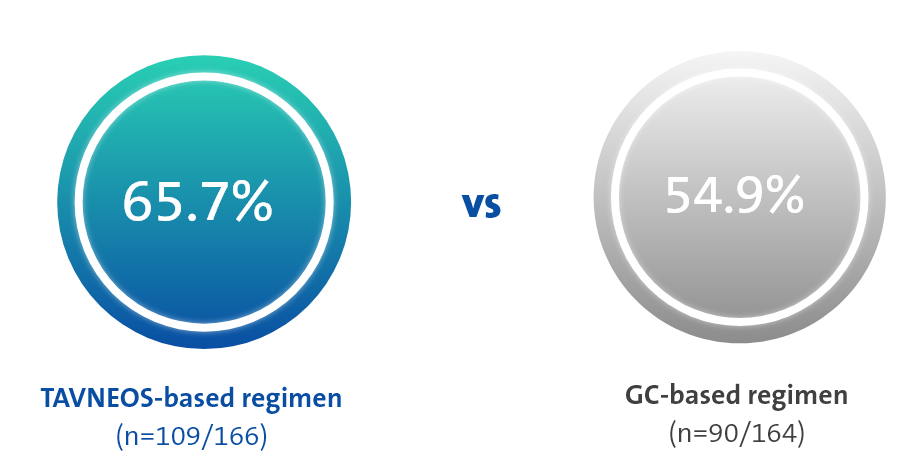
At Week 52, TAVNEOS-based regimen demonstrated a NNT between 8 and 10†
TAVNEOS-based regimen resulted in a lower absolute risk of relapse vs a GC-based regimen over 52 weeks1
Absolute risk of relapse over 52 weeks of treatment1
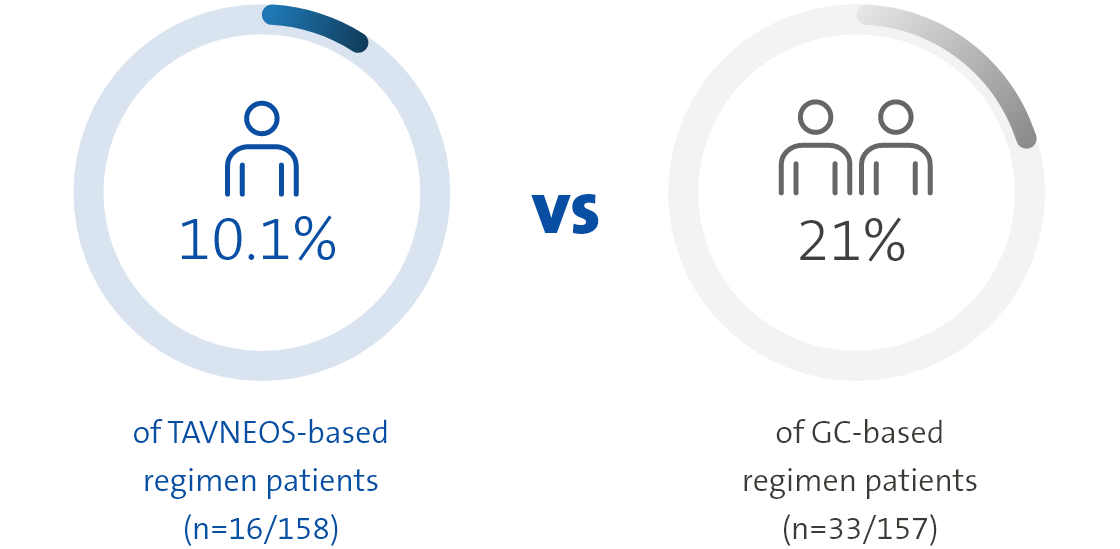

Twice as many patients relapsed in the GC-based regimen vs the TAVNEOS-based regimen1

There was no pre-speci ed plan for adjustment of con dence intervals for multiplicity of the secondary end points; point estimates and 95% con dence intervals only are presented, and no de nite conclusions can be drawn from these data
TAVNEOS®-based regimen demonstrated a 54% relative risk reduction in relapse vs a GC-based regimen over 52 weeks1
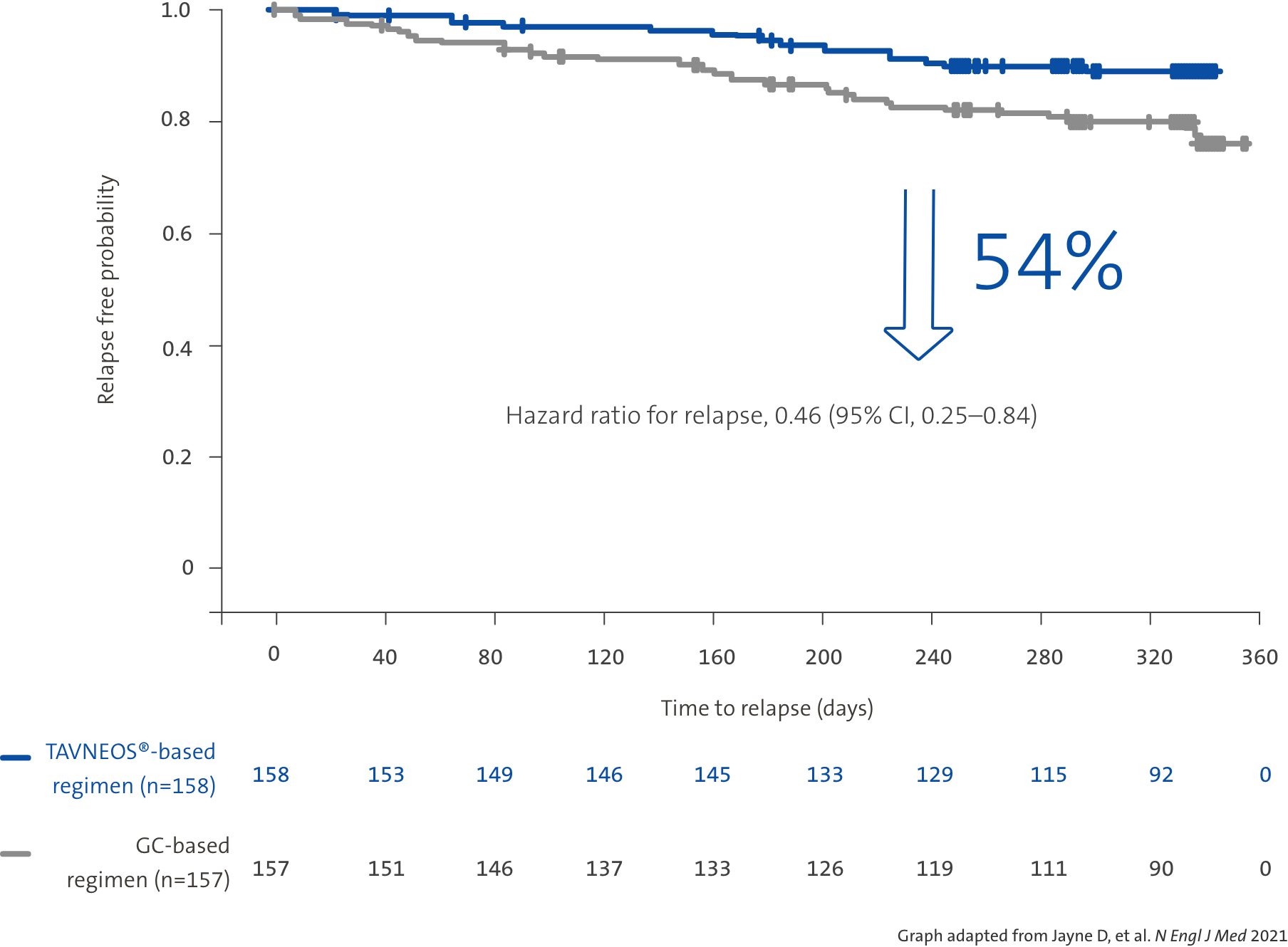
52 week, international, multicentre, active-comparator, randomised, double-blind, double-dummy, controlled, pivotal phase 3 study with patients assigned to receive TAVNEOS 30 mg twice daily (N=166) or prednisone tapering schedule for 20 weeks (60 mg per day tapered to discontinuation by Week 21) (N=164) on the top of either cyclophosphamide (followed by azathioprine) or rituximab.1
TAVNEOS-based regimen patients received NO study-supplied GCs. Patients in both regimens could use non-study supplied GCs throughout the 52-week study at their physician’s discretion for AAV worsening or non-AAV-related reasons. Non-study supplied GCs during the screening had to be tapered to ≤20 mg per day before entering the study, and further tapered to 0 mg by the end of Week 4 of the study.4

| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
*Adult and adolescent GPA/MPA patients participated in this study. For additional details, visit clinicaltrials.gov, study code: NCT02994927
| TAVNEOS-BASED REGIMEN (n=166) | GC-BASED REGIMEN (n=164) | |
|---|---|---|
| AGE (mean±SD) | 61.2±14.6 | 60.5±14.5 |
| MALE no. (%) | 98 (59.0) | 88 (53.7) |
| GRANULOMATOSIS WITH POLYANGIITIS no. (%) | 91 (54.8) | 90 (54.9) |
| MICROSCOPIC POLYANGIITIS no. (%) | 75 (45.2) | 74 (45.1) |
| RELAPSED PATIENTS no. (%) | 51 (30.7) | 50 (30.5) |
| NEWLY DIAGNOSED PATIENTS no. (%) | 115 (69.3) | 114 (69.5) |
| BVAS SCORE no. (%) | 16.3±5.9 | 16.2±5.7 |
| GC USE DURING SCREENING PERIOD no. (%) | 125 (75.3) | 135 (82.3) |
ORGAN INVOLVEMENT no. (%)
|
|
|
| TAVNEOS-BASED REGIMEN (n=166) | |
|---|---|
| AGE (mean±SD) | 61.2±14.6 |
| MALE no. (%) | 98 (59.0) |
| GRANULOMATOSIS WITH POLYANGIITIS no. (%) | 91 (54.8) |
| MICROSCOPIC POLYANGIITIS no. (%) | 75 (45.2) |
| RELAPSED PATIENTS no. (%) | 51 (30.7) |
| NEWLY DIAGNOSED PATIENTS no. (%) | 115 (69.3) |
| BVAS SCORE no. (%) | 16.3±5.9 |
| GC USE DURING SCREENING PERIOD no. (%) | 125 (75.3) |
ORGAN INVOLVEMENT no. (%)
|
|
| GC-BASED REGIMEN (n=164) | |
| AGE (mean±SD) | 60.5±14.5 |
| MALE no. (%) | 88 (53.7) |
| GRANULOMATOSIS WITH POLYANGIITIS no. (%) | 90 (54.9) |
| MICROSCOPIC POLYANGIITIS no. (%) | 74 (45.1) |
| RELAPSED PATIENTS no. (%) | 50 (30.5) |
| NEWLY DIAGNOSED PATIENTS no. (%) | 114 (69.5) |
| BVAS SCORE no. (%) | 16.2±5.7 |
| GC USE DURING SCREENING PERIOD no. (%) | 135 (82.3) |
ORGAN INVOLVEMENT no. (%)
|
|
| Primary | Overview of results |
|---|---|
| Achievement of clinical remission (BVAS 0 and no GC use in previous 4 weeks) | Non-inferior to GC-based regimen (p<0.001 for non-inferiority) |
| Sustained remission at Week 52,defined as clinical remission at Week 26 through to Week 52 |
Superior to GC-based regimen (p=0.007 for superiority) |
| Secondary | Overview of results |
|---|---|
| Change in GC-related toxicity during first 26 weeks according to the GTI | Significant reduction in GTI-CWS at Week 26 (p=0.0002); significant reduction in GTI-AIS at Week 26 (p=0.008) vs GC-based regimen |
| BVAS 0 at Week 4 | Similar number of patients achieved BVAS 0 at Week 4 in both treatment groups |
| Change in health-related QoL scores over 52 weeks as measured by SF-36v2 and EuroQoL-5D-5L | Significant improvements in physical QoL (p=0.002 at Week 26 and p=0.018 at Week 52) and health-related QoL* vs GC-based regimen. Numerical improvement in mental QoL |
| In patients with renal disease at baseline, change over 52 weeks in eGFR, UACR and MCP-1:creatinine ratio | Meaningful improvements in renal function at Week 52 (p=0.029), significant reduction in UACR at Week 4 (p<0.0001); numerically greater reduction in MCP-1:creatinine ratio at Week 52 vs GC-based regimen |
| Relapse (BVAS >0) assessed in a time-to-event analysis | 54% relative reduction in relapse risk over 52 weeks vs GC-based regimen |
| Cumulative organ damage (VDI) | Numerically similar change in VDI from baseline across both treatment groups |
There was no pre-specified plan for adjustment of confidence intervals for multiplicity of the secondary end points; point estimates and 95% confidence intervals only are presented, and no definite conclusions can be drawn from these data
*Health-related QoL was measured using two scores: EQ-5D-5L VAS score and EQ-5D-5L index. Significant improvements in EQ-5D-5L VAS score were observed in the TAVNEOS-based regimen at Week 26 (p=0.05) and Week 52 (p=0.002) vs GC-based regimen. With the EQ-5D-5L index, numerical improvements were observed at Week 26, and significant improvements were observed at Week 52 (p=0.009) in the TAVNEOS-based regimen vs GC-based regimen5
TAVNEOS®-based regimen demonstrated meaningful improvements in eGFR1
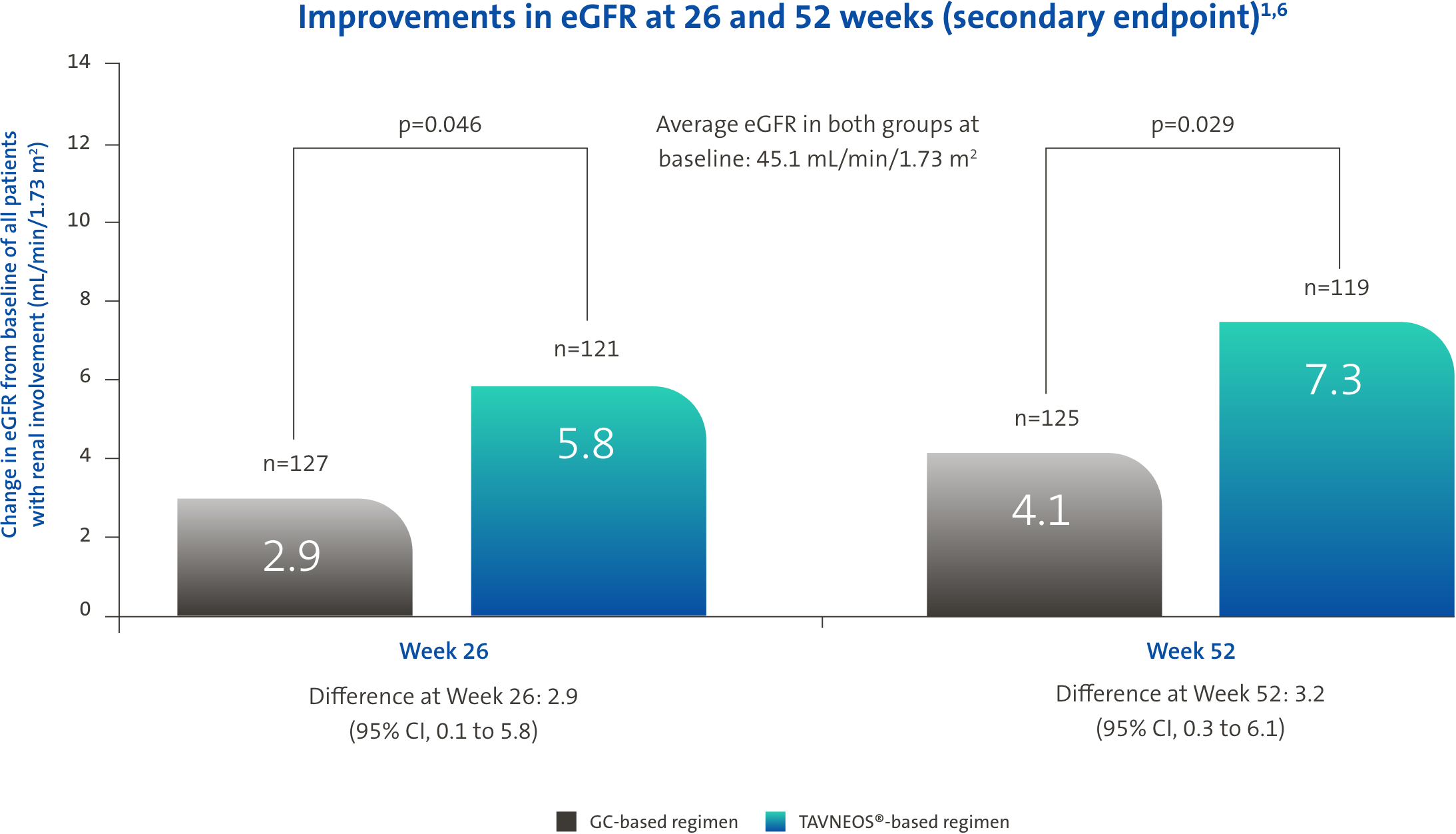

mL/min/1.73 m2 eGFR increase in TAVNEOS®-based regimen (n=52) vs +8.2 mL/min/1.73 m2 in GC-based regimen (n=48) at Week 52 (p=0.005) (Difference 5.6, 95% CI, 1.7 to 9.5)

mL/min/1.73 m2 eGFR increase in TAVNEOS®-based regimen (n=27) vs +7.7 mL/min/1.73 m2 in GC-based regimen (n=23) at Week 52 (p=0.003) (Difference 8.4, 95% CI, 2.9 to 13.8)
There was no pre-specified plan for adjustment of confidence intervals for multiplicity of the secondary end points; point estimates and 95% confidence intervals only are presented, and no definite conclusions can be drawn from these data
TAVNEOS®-based regimen demonstrated a significant reduction in GC toxicity1,4

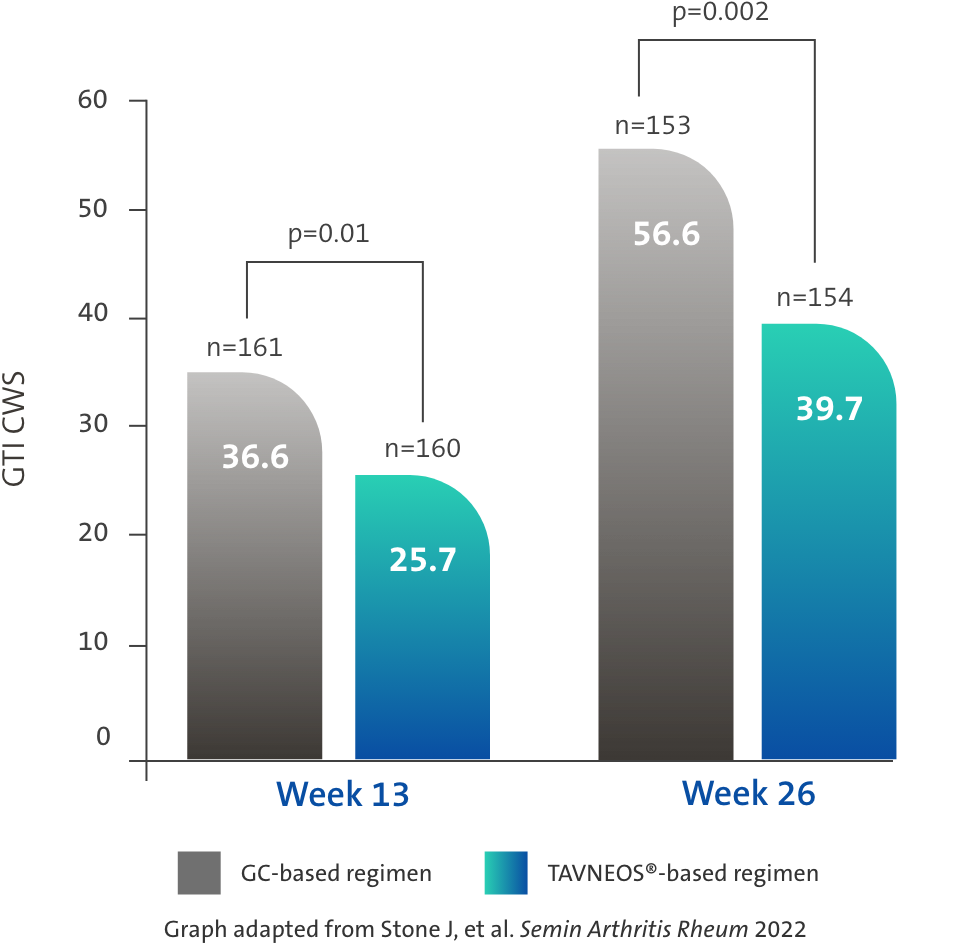
Difference at Week 13: -11.0 (95% CI, -19.7 to -2.2)
Difference at Week 26: -16.8 (95% CI, -25.6 to -8.0)

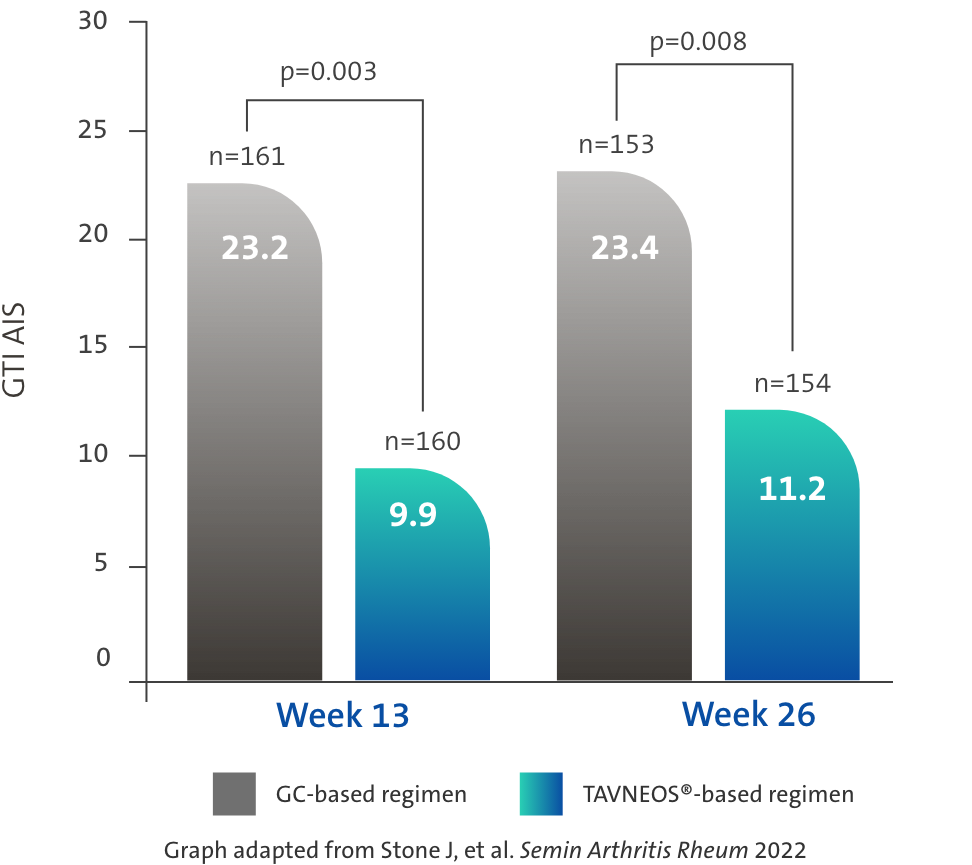
Difference at Week 13: -13.3 (95% CI, -22.2 to -4.4)
Difference at Week 26: -12.1 (95% CI, -21.1 to -3.2)
There was no pre-specified plan for adjustment of confidence intervals for multiplicity of the secondary end points; point estimates and 95% confidence intervals only are presented, and no definite conclusions can be drawn from these data
TAVNEOS®-based regimen demonstrated significant improvements in physical and health-related domains of QoL1,3,5
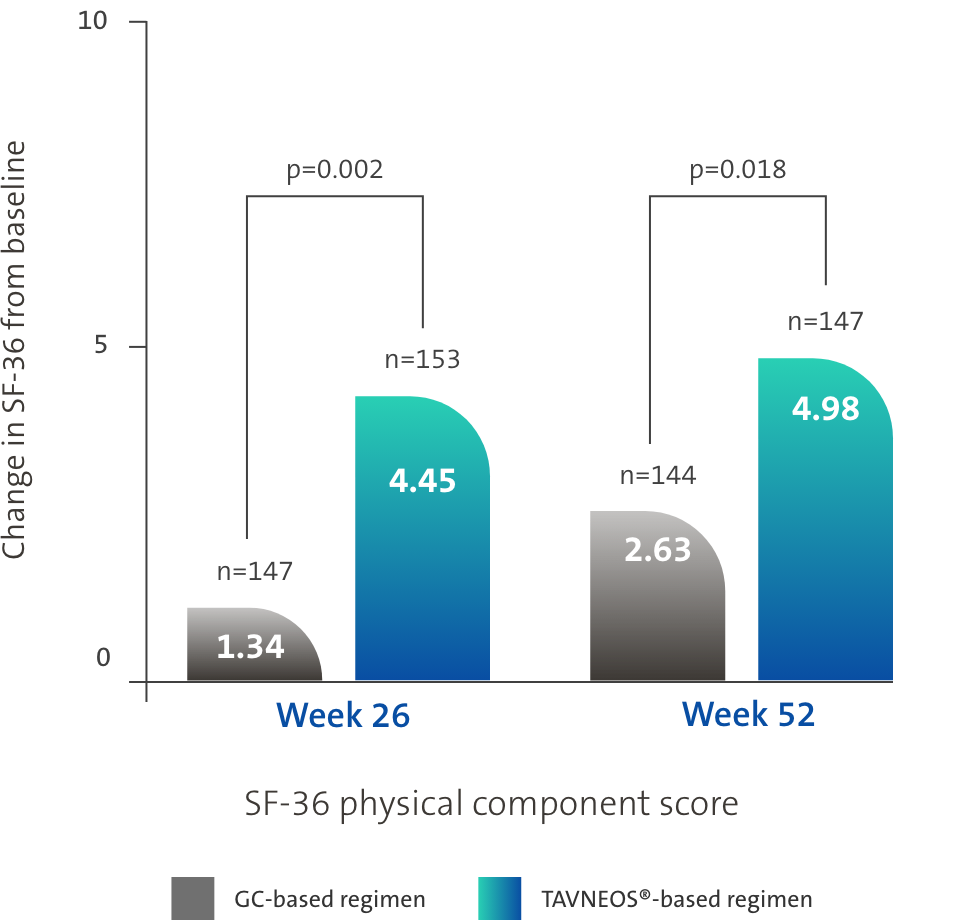
Difference at Week 26: 3.10 (95% CI, 1.17 to 5.03)
Difference at Week 52: 2.35 (95% CI, 0.40 to 4.31)
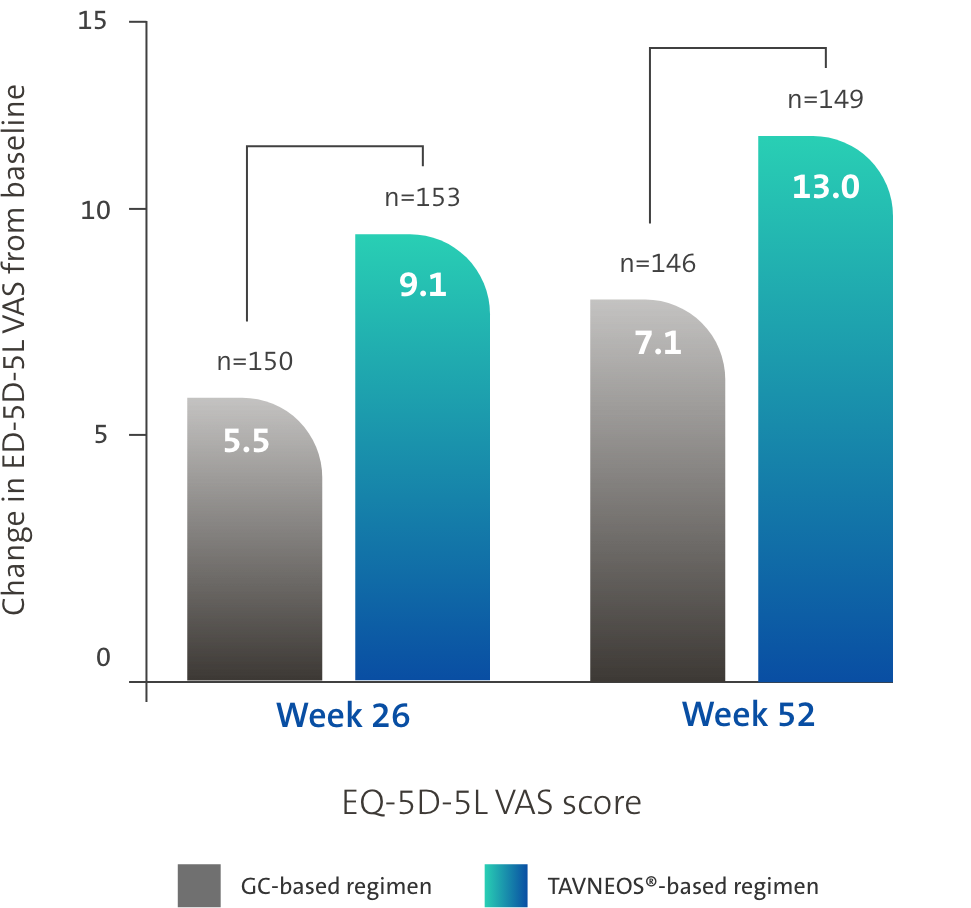
Difference at Week 26: 3.6 (95% CI, -0.1 to 7.2)
Difference at Week 52: 5.9 (95% CI, 2.3 to 9.6)
ADVOCATE is the first study to show a significant difference in the QoL between treatment arms1,3,5,8–10
There was no pre-specified plan for adjustment of confidence intervals for multiplicity of the secondary end points; point estimates and 95% confidence intervals only are presented, and no definite conclusions can be drawn from these data
*Clinical remission in the ADVOCATE study was defined as a BVAS 0 and no glucocorticoid use in previous 4 weeks.1
The NNT was calculated using https://www.statology.org/confidence-interval-difference-in-proportions-calculator/ to determine the confidence intervals for crude difference in proportions. At Week 52, the response rates were 65.7% in the TAVNEOS-based regimen and 54.9% in the GC-based regimen. This led to a crude result of NNT = 1/(0.657-0.549) = 9.26, rounded to 10 patients. Using the adjusted model and the estimated common difference, the NNT = 1/0.125 = 8. In conclusion, the NNT is between 8 and 10 patients at Week 52.1
‡A numerical improvement was seen in mental QoL.5
§With the EQ-5D-5L index, numerical improvements were observed at Week 26, and significant improvements were observed at Week 52 (p=0.009) in the TAVNEOS-based regimen vs GC-based regimen.5
AAV, ANCA-associated vasculitis; AIS, aggregate improvement score; ANCA, anti-neutrophil cytoplasmic antibody; BVAS, Birmingham Vasculitis Activity Score; CD19, cluster of differentiation 19; CI, confidence interval; CKD, chronic kidney disease; CWS, Cumulative Worsening Score; CYC,, cyclophosphamide; eGFR,, gestimated glomerular filtration rate; EQ-5D-5L, EuroQol 5-dimension 5-level; GC, glucocorticoid; IV, intravenous; MCP-1, monocyte chemoattractant protein-1; MOA, mechanism of action; MPA, microscopic polyangiitis; MPO, myeloperoxidase; NNT, number-needed-to-treat; PR3, proteinase-3; QoL, quality of life; UACR, urine albumin-to-creatinine ratio; VAS, Visual Analogue Scale; VDI Vasculitis Damage Index.
1. Jayne D, et al. N Eng J Med 2021; 384(7): 599–609. 2. TAVNEOS EU SmPC January 2022. 3. Bekker P, et al. PLoS One 2016;11(10):e0164646 4. Jayne D, et al. N Engl J Med 2021;384(7):599–609. [Supp Appendix]. 5. Vifor Pharma (2020). Data on File. 6. Jayne D, et al. J Am Soc Nephrol 2021:32 7. NHS (2019). Chronic Kidney Disease. Available at: https://www.nhs.uk/conditions/kidney-disease/diagnosis/. Date accessed: March 2023. 8. Cortazar F, et al. Kidney Int Rep 2023. Available at: https://doi.org/10.1016/j.ekir.2023.01.039. Date accessed: March 2023. 9. Stone J, et al. Semin Arthritis Rheum 2022;55:152010. 10. McDowell P, et al. J Allergy Clin Immunol Pract 2021;9(1):365–72. 11. Stone JH, et al. N Engl J Med 2010;363(3):221–32. 12. Walsh M, et al. N Engl J Med 2020;382(7):622–31. 13. Charles P, et al. Ann Intern Med 2020;173(3):179–87.