First-in-class targeted treatment
TAVNEOS is the only targeted therapy for AAV (GPA/
MPA) to address a key driver of vascular
inflammation in the alternative complement pathway1,2
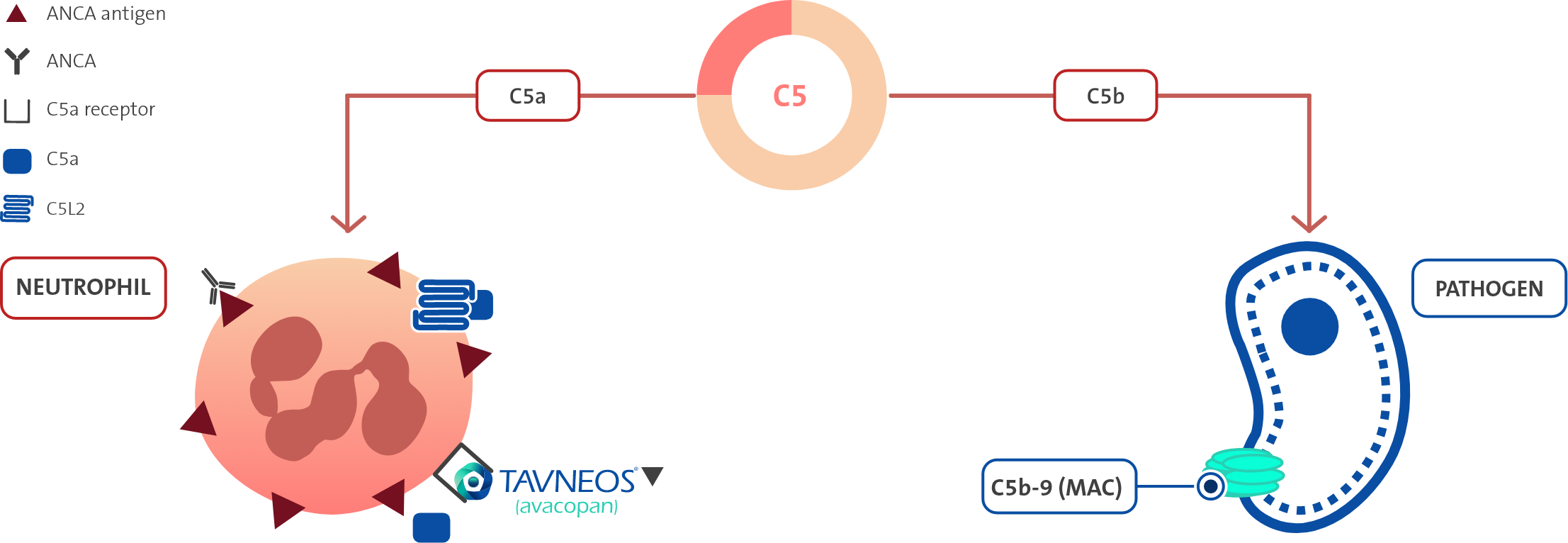
TAVNEOS reduces the pro-inflammatory effects of C5a, including:1
- Neutrophil activation and migration
- Adherence to sites of small blood vessel inflammation
- Vascular endothelial cell retraction and permeability
TAVNEOS does not block the production of C5b, which is essential for the formation of the membrane attack complex (MAC)1
Activation of the alternative complement pathway plays a key role in AAV, and no established therapies address this key driver of vascular inflammation1,3–8
Already recognised in the EULAR 2022 recommendations
The EULAR recommendations for the management of ANCA-associated vasculitis: 2022 update recognised the ability of a TAVNEOS-based regimen to improve disease control, and the potential to lower GC toxicity and improve renal function9,10
EULAR 2022 RECOMMENDATION LEVEL OF EVIDENCE 1b:
TAVNEOS (avacopan) in combination with RTX or CYC may be considered for induction of remission in GPA or MPA, as part of a strategy to substantially reduce exposure to GCs9
Consider TAVNEOS in subgroups likely to have enhanced benefit vs GC therapy:9
- patients at risk of development or worsening of GC-related adverse effects and complications
- patients with active glomerulonephritis and rapidly deteriorating kidney function
No data on the use of TAVNEOS beyond 1 year, so longer-term use cannot be recommended9
In ADVOCATE, remission was sustained to Week 52 (primary endpoint) at a higher rate in the TAVNEOS-based regimen (65.7%) vs GC-based regimen (54.9%) so TAVNEOS appears to have efficacy for maintenance of remission 9
 Please rotate your device
Please rotate your device
Understand more about the pathogenesis of AAV and how TAVNEOS works
AAV, ANCA-associated vasculitis; ANCA, anti-neutrophil cytoplasmic antibody; C5a, complement component 5a; C5aR, C5a receptor; C5b, complement component 5b; C5L2, C5a receptor-like 2; CYC, cyclophosphamide; EULAR, European Alliance of Associations for Rheumatology; GC, glucocorticoid; GPA, granulomatosis with polyangiitis; MAC, membrane attack complex; MOA, mechanism of action; MPA, microscopic polyangiitis; RTX, rituximab.
References
1. Bekker P, et al. PLoS ONE 2016;11(10):e0164646. 2. Thurman JM, Holers VM. J Immunol 2006;176(3):1305–10. 3. Hutton HL, et al. Semin Nephrol 2017;37(5):418–3. 4. Jennette JC, Nachman PH. Clin J Am Soc Nephrol 2017;12(10):1680–91. 5. Al-Hussain T, et al. Adv Anat Pathol 2017;24(4):226–34. 6. Lamprecht P, et al. EMJ Rheumatol 2021;8(1):36–42. 7. Liberman AC, et al. Front Endocrinol (Lausanne) 2018;9:235. 8. Nozaki Y. Front Immunol 2021;12:631055. 9. Hellmich B, et al. Ann Rheum Dis 2023;0:1–18. 10. Jayne D, et al. N Engl J Med 2021;384(7):599–609.


 Scroll down
Scroll down